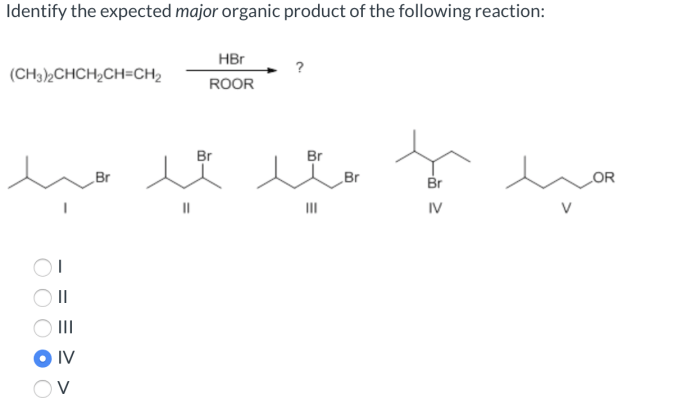
Draw bond-line structures for all constitutional isomers of c4h10 – Embarking on an exploration of bond-line structures for all constitutional isomers of C4H10, this comprehensive guide unveils a captivating journey into the realm of organic chemistry. Delving into the intricacies of structural isomerism, we unravel the significance of constitutional isomers, providing a thorough understanding of their distinct characteristics and properties.
Unveiling the intricacies of bond-line structures, this guide illuminates their purpose and conventions, empowering readers with the knowledge to effectively represent organic molecules. Through a step-by-step approach, we navigate the intricacies of drawing bond-line structures, equipping readers with a valuable tool for visualizing and comprehending molecular structures.
Structural Isomerism
Structural isomerism refers to the phenomenon where compounds with the same molecular formula have different structural arrangements of their atoms. This difference in structure leads to distinct physical and chemical properties.
Constitutional isomers, a type of structural isomer, are compounds that have the same molecular formula but differ in the way their atoms are connected. For example, butane (C4H10) has two constitutional isomers: n-butane and isobutane.
Bond-Line Structures
Bond-line structures are simplified representations of molecules that use lines to represent bonds and circles or dots to represent carbon atoms. These structures provide a clear and concise way to visualize the connectivity of atoms within a molecule.
- Start by drawing a circle or dot for each carbon atom in the molecule.
- Connect the carbon atoms with lines to represent the bonds between them.
- Add hydrogen atoms to each carbon atom to satisfy its valency.
- Remove any unnecessary lines or circles.
Constitutional Isomers of C4H10
The molecular formula C4H10 can form two constitutional isomers:
- n-Butane:A straight-chain hydrocarbon with the structure CH3-CH2-CH2-CH3
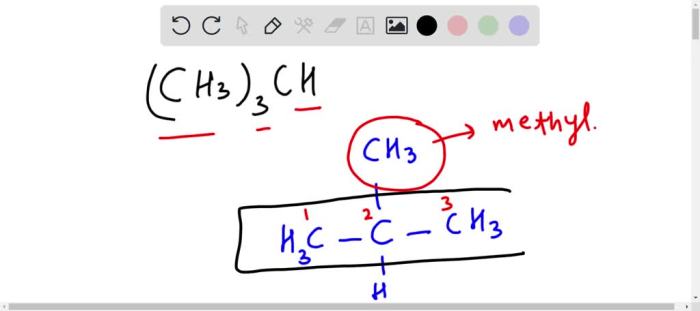
- Isobutane:A branched-chain hydrocarbon with the structure (CH3)3-CH
The bond-line structures for these isomers are:
- n-Butane: CH3-CH2-CH2-CH3
- Isobutane: (CH3)3-CH
Properties and Applications
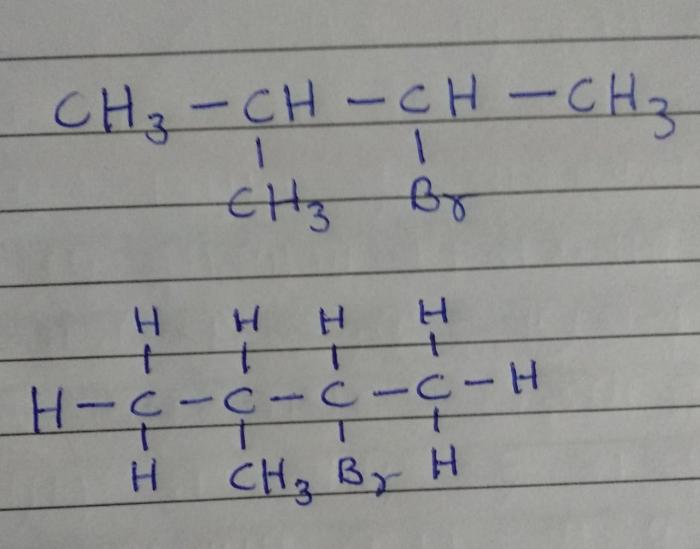
The constitutional isomers of C4H10 have slightly different physical and chemical properties.
- n-Butane:A colorless gas with a boiling point of -0.5°C and a melting point of -138.3°C. It is used as a fuel and as a feedstock for the production of other chemicals.
- Isobutane:A colorless gas with a boiling point of -11.7°C and a melting point of -160°C. It is used as a refrigerant, a propellant, and as a feedstock for the production of other chemicals.
Table of Isomers
| Bond-Line Structure | Name | Boiling Point (°C) | Melting Point (°C) |
|---|---|---|---|
| CH3-CH2-CH2-CH3 | n-Butane | -0.5 | -138.3 |
| (CH3)3-CH | Isobutane | -11.7 | -160 |
Essential FAQs: Draw Bond-line Structures For All Constitutional Isomers Of C4h10
What is the molecular formula of C4H10?
C4H10 represents the molecular formula for butane, a four-carbon alkane with the chemical structure CH3(CH2)2CH3.
How many constitutional isomers are there for C4H10?
There are two constitutional isomers of C4H10: butane and isobutane. Butane has a linear carbon chain, while isobutane has a branched carbon chain.
What are the physical properties of butane?
Butane is a colorless, flammable gas at room temperature and pressure. It has a boiling point of -0.5 °C and a melting point of -138.3 °C.